MISSION STATEMENT:
The overall goal of the Pepper Study Registry (PSR) is to guarantee the proper and long-term access to, preservation and management of data resources to be used for conducting future studies by investigators with an interest in aging-related research.- The PSR provides meta-data for self-deposited aging studies from Pepper Older American Independence Centers (OAICs).
- The PSR provides data resources to the Designated Community (researchers and practitioners related to aging) by curating, preserving, and disseminating raw data that are deposited into the repository.
PSR WORKFLOW:
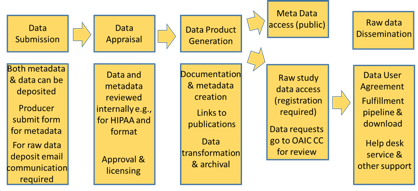
Download to read full details on the PSR Workflow
PSR DATA INTEGRITY & AUTHENTICITY:
Describes the policy for data integrity and authenticity for the PSR. Data integrity and authenticity is managed within the PSR system as part of the processes for data deposit, database change management, and data access. The integrity and authenticity of the data also is recorded as part of the documentation of changes to the data.Download to read full details on PSR Data Integrity and Authenticity
PSR DATA PRESERVATION POLICY:
Describes the PSR's data preservation policy to the data depositors and users of the PSR. The PSR follows the general functions for data preservation described by the Open archival information system (OAIS) framework [1], including data ingest, storage, management, access, dissemination, and migration. The PSR recognizes the varying levels of responsibility in preserving each deposited item. Therefore, this policy of the PSR preservation plan is made available to all PSR staff and to the users of the PSR.Download to read full details on the PSR Data Preservation Statement
PSR REVIEW COMMITTEE:
The PSR Review Committee includes a body of experts, both intramural and outside of the institution (Wake Forest University School of Medicine) for providing technical review and consultation for the PSR. The goal is to ensure that the PSR is up to date on compliance with principles for a standard, modern data registry and repository.Download to read full details on the PSR Review Committee
PSR CONTINUED SUPPORT:
Link to NIA RePORTERContact the Registry Coordinator at (336) 713-7126 or PepperCenter@WakeHealth.edu for additional information.
How to search: Enter any single word, or combination of words using " + " (space plus space) in between. As an example, to search for both male and female with a condttion of obesity that have DEXA, enter "DEXA + Obesity + M/F". Clicking a column header will sort the data by that column's data.
Notes: Most studies will have some available demographic and many will have laboratory data. An asterisks (*) indicate study collected by not available in the database.
Notes: Most studies will have some available demographic and many will have laboratory data. An asterisks (*) indicate study collected by not available in the database.
| Study (PI) | Gender | Age | Disease/ Condition |
N= | Design / Intervention |
Biospecimen | Imaging | Physical | Activity Monitoring |
Cognitive / Depression |
|---|---|---|---|---|---|---|---|---|---|---|
|
Functional near-infr...
(Huppert, Pittsburgh) |
M / F | 19 | cross-sectional cohort |
|
DSST
Stroop |
|||||
|
This study measured ...
(Hergenroeder, Pittsburgh) |
M / F | 69 -98 | 43 | cross-sectional cohort |
|
ActivPAL
|
||||
|
7 activity monitors ...
(Hergenroeder, Pittsburgh) |
M / F | 69 -98 | 43 | cross-sectional cohort |
|
Actigraph (Wrist)
Actigraph (Hip) ActivPAL Fitbit |
||||
|
ADAPT
(Messier, WFU) |
M / F | 60 + | knee osteoarthritis | 316 | exercise / diet 18 mos |
|
Gait Speed
PAT-D |
MMSE
CES-D |
||
|
AML
(Klepin, WFU) |
M / F | 60 + | AML | 81 | Parallel Assignment 24 weeks |
|
Grip Strength
SPPB PAT-D Knee Extensor |
3MSE
CES-D |
||
|
APPLE
(Nicklas, WFU) |
M / F | 65 -79 | Arthritis, OA, weight loss | 40 | weight loss: no vest vs weighted vest 22 weeks |
Serum Plasma Adipose |
DEXA
|
Gait Speed
ESPPB Leg Press Knee Extensor |
MoCA
CES-D |
|
|
Bicarb
(Petrovic, WFU) |
M / F | 65 + | Kidney disease / disability | 80 | 3 months RCT oral bicarbonate supplement vs placebo |
Serum Plasma Urine |
DEXA
|
Gait Speed
SPPB |
MoCA
|
|
|
BRJS
(Miller, WFU) |
M / F | 60 -84 | 27 | randomized, double blind, placebo-controlled trial |
Plasma |
CT
DEXA |
Gait Speed
Knee Extensor |
|||
|
CLIP
(Rejeski, WFU) |
M / F | 60 -79 | Overweight/obese; CVD or METs | 288 | 18-month RCT Caloric Restriction and/or Aerobic Exercise |
Serum Plasma |
DEXA
|
Gait Speed
SPPB PAT-D |
||
|
CLIP II
(Rejeski, WFU) |
M / F | 60 -79 | cardiovascular disease | 252 | Weight Loss alone, aerobic exercise training +Weight Loss or resistance exercise training +Weight Loss 18 mos |
DEXA
*
|
SPPB
*
Knee Extensor * |
Actigraph (Hip)
*
|
||
|
COCOA-PAD II
(McDermott, NW) |
M / F | 55 + | Peripheral artery disease | 190 | RCT cocoa flavanols |
Serum Plasma DNA |
MRI
|
Gait Speed
SPPB CPET |
Actigraph (Hip)
|
MMSE
|
|
DEMO
(Nicklas, WFU) |
F | 50 -70 | Abdominal obesity | 112 | 5-month RCT Caloric Restriction vs. CR + Aerobic Exercise |
Serum Plasma Adipose DNA |
DEXA
|
Grip Strength
SPPB PAT-D |
MMSE
CES-D |
|
|
EFFECT - Pilot
(Morris, WFU) |
M / F | 18 + | ICU patients | 100 | Cross-sectional Intensive physical therapy vs. Usual Care |
Serum Plasma DNA |
|
|||
|
EMPOWER
(Nicklas, WFU) |
M / F | 65 -85 | Obesity | 189 | Randomized to 3 treatment arms 18 mos |
Serum Plasma |
DEXA
|
Gait Speed
Grip Strength ESPPB |
ActivPAL
*
|
DSST
MoCA CES-D |
|
EVIDENCE
(Houston, WFU) |
M / F | 65 + | Vitamin D Insufficient | 200 | Vitamin D vs Placebo 12 month |
Serum Muscle |
|
Gait Speed
Grip Strength SPPB ESPPB Leg Press Knee Extensor CHAMPS Postural Sway Gait Rite |
DSST
MoCA Story Recall * CES-D |
|
|
EVIDENCE Sub Study
(Molina, WFU) |
M / F | 65 + | Vitamin D Insufficient | 40 | Vitamin D & Muscle Function 4 months |
Serum Plasma |
|
Gait Speed
Grip Strength SPPB ESPPB Leg Press |
MoCA
|
|
|
FIT (F/U Infinite)
(Nicklas, WFU) |
M / F | 65 -80 | obesity, follow up to INFINITE Study | 0 | 18 mos |
Plasma DNA |
|
Gait Speed
ESPPB CHAMPS * |
DSST
MMSE CES-D |
|
|
FLOW
(Hundley, WFU) |
M / F | 50 + | Aging/Heart Failure | 30 | Cross-sectional N/A |
Serum Plasma |
|
Grip Strength
SPPB PAT-D |
||
|
GIFT II
(McDermott, NW) |
M / F | 55 + | Peripheral artery disease | 30 | RCT unacylated ghrelin |
Serum Plasma DNA |
MRI
|
Gait Speed
SPPB CPET |
MMSE
|
|
|
GO MET
(Ding, WFU) |
M / F | 65 -79 | CAD / Cancer / MCI / Physcial Function Decline | 30 | Crossover Metformin vs Placebo 24 months |
Serum Plasma DNA |
|
Gait Speed
Grip Strength ESPPB PAT-D Leg Press Knee Extensor * |
DSST
MoCA |
|
|
Healthy Aging
(Kitzman, WFU) |
M / F | 60 + | Healthy controls | 61 | Cross-sectional N/A |
Serum Plasma Urine Muscle DNA |
DEXA
|
Gait Speed
Grip Strength SPPB PAT-D Leg Press Knee Extensor CHAMPS |
ActivPAL
*
|
MMSE
Rey AVLT * Trail Making * |
|
HI-PAD
(McDermott, NW) |
M / F | 55 + | Peripheral artery disease | 39 | RCT VM202 |
Serum Plasma DNA |
CT
MRI |
Gait Speed
SPPB CPET |
MMSE
|
|
|
HOPE - Pilot
(Houston, WFU) |
M / F | 55 + | Obesity | 54 | Exercise Study Follow up / Feasibility Study n/a |
DEXA
|
Gait Speed
Grip Strength ESPPB Leg Press Knee Extensor * |
DSST
*
MoCA Rey AVLT * TICS * Trail Making * CES-D * |
||
|
I'M FIT
(Nicklas, WFU) |
M / F | 65 -79 | Obese; at risk for disability | 130 | 5-month RCT Resistance Training (RT) vs. Resistance Training (RT) + Caloric Restriction |
Serum Plasma Muscle Adipose |
CT
DEXA |
Gait Speed
Grip Strength SPPB ESPPB PAT-D Leg Press Knee Extensor |
MMSE
|
|
|
IDEA
(Messier, WFU) |
M / F | 55 + | Overweight/obese; Knee OA | 450 | 18-month RCT Caloric Restriction vs. Aerobic Exercise vs. CR+AEX |
Serum Plasma Urine DNA |
CT
DEXA |
Gait Speed
Grip Strength SPPB PAT-D Leg Press |
ActivPAL
*
|
DSST
MMSE 3MSE CES-D |
|
INFINITE
(Nicklas, WFU) |
M / F | 65 -79 | Obese | 180 | 5-month RCT Aerobic Exercise vs AEX + Hi Caloric Restriction vs AEX + Low Caloric Restriction |
Serum Plasma Adipose DNA |
DEXA
|
Gait Speed
SPPB PAT-D |
RT3
*
|
DSST
MMSE Rey AVLT * COWA * Stroop * CES-D |
|
INTERCEDE
(McDermott, NW) |
M / F | 55 + | Peripheral artery disease | 230 | RCT Intermittent Pneumatic Compression (IPC) with and without exercise |
Serum Plasma DNA |
MRI
|
Gait Speed
SPPB CPET |
Actigraph (Wrist)
|
MMSE
Stroop |
|
LEAN
(Nickals, WFU) |
M / F | Obesity | 0 |
Serum Plasma Muscle |
CT
DEXA |
Gait Speed
Grip Strength ESPPB PAT-D Leg Press Knee Extensor |
MMSE
|
|||
|
LIFE - Pilot
(Kritchevsky, WFU) |
M / F | 70 -89 | At risk for disability | 424 | 12-month RCT Aerobic + Resistive Exercise vs. Control |
Serum Plasma DNA |
DEXA
*
|
Gait Speed
Knee Extensor * |
CES-D
*
|
|
|
LOSE-IT
(Miller, WFU) |
M / F | 18 + | Bariatric surgery patients | 42 | Cross-sectional N/A |
Serum Plasma Muscle Adipose |
CT
DEXA |
SPPB
PAT-D Knee Extensor * |
CES-D
|
|
|
MAT-PAC
(Kim, WFU) |
M / F | 65 + | Mobility | 0 | observational |
|
||||
|
Medifast
(Beavers, WFU) |
M / F | 65 -79 | Obesity | 124 | Weight Loss (High Protein Group) / Weight Stable group 24 mos |
Serum Plasma Urine |
DEXA
*
|
Gait Speed
Grip Strength ESPPB PAT-D Leg Press |
Actigraph (Wrist)
*
|
DSST
MoCA CES-D |
|
MidCog
(Wolf, NW) |
M / F | 40 -64 | 1200 | observational longitudinal cohort study N/A |
|
Gait Speed
Grip Strength |
Actigraph (Wrist)
|
MoCA
Story Recall |
||
|
MORPH
(Brooks, WFU) |
M / F | 55 -85 | Obesity / Pain | 35 | (2) a two group randomized controlled pilot trial (RCT) in 35 obese (BMI=30-45 kg/m2), low-active, older (55-85 years) adults with chronic pain randomized to either 12-weeks of active intervention or a wait-list control. 12 weeks |
|
ESPPB
|
MoCA
CES-D |
||
|
MOW Vitamin D
(Houston, WFU) |
M / F | 65 + | Vitamin D Insufficient | 68 | 100,000 IU OF VITAMIN D VS PLACEBO (400 IU VIT E) AND FALLS ASSESSMENT 5 month Randomized Control Trial |
Serum Plasma |
|
PAT-D
|
||
|
NICE
(McDermott, NW) |
M / F | 18 + | Peripheral artery disease | 90 | RCT Nicotinamide riboside, resveratrol |
Serum Plasma DNA |
|
Gait Speed
SPPB CPET |
MMSE
|
|
|
OPTIFAST
(Ard, WFU) |
M / F | 64 + | Obesity / Disability | 88 | high intensity weight loss protocol VS a moderate intensity weight loss protocol 26 weeks |
DEXA
|
Gait Speed
ESPPB Knee Extensor |
DSST
MoCA |
||
|
OPTIMA
(Kritchevsky, WFU) |
M / F | 65 -79 | Overweight/obese; at risk for disability | 88 | 6-month RCT Caloric Restriction + Pioglitazone vs. CR + Resistance Exercise |
Serum Plasma Muscle |
CT
DEXA |
Gait Speed
Grip Strength SPPB ESPPB PAT-D Leg Press Knee Extensor * CHAMPS |
MMSE
CES-D |
|
|
PA AML
(Klepin, WFU) |
M / F | 60 + | Acute Myeloid Leukemia | 70 | Interventional Clinical Trial Parallel Assignment |
|
Grip Strength
SPPB PAT-D |
DSST
CES-D |
||
|
PACT
(Miller, WFU) |
M / F | 60 + | Overweight/obese; Knee OA | 84 | 6-month RCT Caloric Restriction + Exercise vs. Control |
Serum Plasma Muscle DNA |
DEXA
|
Gait Speed
PAT-D * |
||
|
PAIN
(Brooks, WFU) |
M / F | 65 + | chronic pain | 33 | observational 3 months |
|
SPPB
PAT-D |
|||
|
PART - Transplant
(Hartmann, WFU) |
M / F | 60 + | Renal Transplant | 30 | physical activity 3 months |
Serum Urine |
DEXA
|
Gait Speed
Grip Strength SPPB PAT-D Leg Press CHAMPS |
Actigraph (Hip)
*
|
MMSE
CES-D |
|
PERMET
(McDermott, NW) |
M / F | 18 + | Peripheral artery disease | 212 | RCT metformin |
Serum Plasma DNA |
|
Gait Speed
SPPB CPET |
MMSE
|
|
|
PIE
(Kitzman, WFU) |
M / F | 60 + | Diastolic heart failure | 80 | 12-month RCT ACE inhibitor use vs Placebo |
Serum Plasma Muscle DNA |
DEXA
|
Grip Strength
SPPB PAT-D |
||
|
PIE II
(Kitzman, WFU) |
M / F | 60 + | Diastolic heart failure | 80 | 12-month RCT Spironolactone vs Placebo |
Serum Plasma Muscle DNA |
DEXA
|
Grip Strength
SPPB PAT-D |
||
|
POWER
(Marsh, WFU) |
M / F | 65 + | At risk for disability | 45 | 3-month RCT Strength training vs. Power training vs. Control |
Serum Plasma Muscle |
DEXA
|
SPPB
PAT-D |
CES-D
|
|
|
PREDICT
(Hundley, WFU) |
M / F | 55 -85 | At risk for CHF | 608 | Longitudinal cohort N/A |
Serum DNA |
MRI
|
Gait Speed
Grip Strength SPPB PAT-D Leg Press |
||
|
PROVE
(McDermott, NW) |
M / F | 18 -90 | Peripheral artery disease | 212 | RCT Weight loss, Exercise |
Serum Plasma DNA |
|
Gait Speed
SPPB CPET |
Actigraph (Wrist)
|
MMSE
|
|
RAINS
(Kritchevsky, WFU) |
M / F | 65 -85 | Overweight/obese; at risk for disability | 67 | 4-month intervention Nutrition (protein) supplementation |
Serum Plasma |
|
|||
|
REACT II
(Berry, WFU) |
M / F | 37 -95 | COPD | 200 | 12-month RCT Aerobic Exercise |
Serum Plasma DNA |
DEXA
|
Grip Strength
SPPB PAT-D Knee Extensor * |
CES-D
|
|
|
REACT III
(Berry, WFU) |
M / F | 45 -80 | COPD | 32 | 4-month RCT Resistance Exercise vs. Control |
Serum Plasma Muscle DNA |
|
|||
|
SECRET
(Kitzman, WFU) |
M / F | 60 + | Overweight/obese; heart failure | 100 | 5-month RCT Caloric Restriction vs. Aerobic Exercise vs. CR+AEX |
Serum Plasma Muscle DNA |
DEXA
|
Gait Speed
Grip Strength SPPB PAT-D Leg Press |
ActivPAL
*
|
|
|
SECRET-II
(Kitzman, WFU) |
M / F | 60 + | HFpEF | 88 | CR+AT vs CR+AT+FT 24 weeks |
MRI
DEXA * |
Gait Speed
SPPB * |
CES-D
*
|
||
|
SILVER
(Beavers, WFU) |
M / F | 60 -79 | Obesity | 24 | hypocaloric dietary intervention soy based meal replacemts vs animal protein based MR 3 months |
Serum Muscle DNA |
CT
DEXA |
Gait Speed
Grip Strength SPPB ESPPB * Knee Extensor |
MMSE
|
|
|
Skeletal MRI
(Kitzman, WFU) |
M / F | 60 + | Exercise Intolerance, Heart failure, preserved ejection fraction | 20 | This study will enroll 20 participants. 10 participants will be 60 years and older, have been diagnosed with HFpEF, and suffer exercise intolerance. The remaining 10 participants will be in the age and sex matched control group. 4 weeks |
Serum Plasma |
MRI
|
Gait Speed
Grip Strength SPPB Knee Extensor |
CES-D
*
|
|
|
START
(Messier, WFU) |
M / F | 50 + | Arthritis | 372 | randomized trial 18 mos |
|
MoCA
*
|
|||
|
SWALLOW
(Butler, WFU) |
M / F | 18 -100 | swallowing disorder | 220 |
CT
|
Gait Speed
Grip Strength SPPB PAT-D |
DSST
MMSE CES-D |
|||
|
TRAIN
(Kritchevsky, WFU) |
M / F | 55 + | Heart Failure | 294 |
Serum Plasma Urine |
DEXA
|
Grip Strength
SPPB PAT-D |
CES-D
|
||
|
UNITS
(Parker-Autry, WFU) |
F | 70 + | Urinary Incontinence | 71 | Interventional (Clinical Trial) Parallel Assignment |
Urine |
DEXA
*
|
Gait Speed
Grip Strength ESPPB PAT-D Knee Extensor Postural Sway |
MoCA
CES-D |
|
|
UPLIFT
(Houston, WFU) |
M / F | 65 -85 | Obesity | 187 | Interventional (Clinical Trial) 18 mos |
CT
DEXA |
Gait Speed
Grip Strength SPPB * ESPPB Knee Extensor CHAMPS |
DSST
MoCA CES-D |
||
|
Vitamin D Supplement
(Houston, WFU) |
M / F | 70 -89 | Vitamin D insufficient | 26 | 4-month RCT Vitamin D + Calcium supplementation vs. calcium only |
Serum Plasma Muscle |
DEXA
|
Gait Speed
Grip Strength SPPB PAT-D Leg Press Knee Extensor * Postural Sway * |
DSST
*
MoCA * MMSE Story Recall * CES-D * |
|
|
WALCS
(McDermott, NW) |
M / F | 50 + | Peripheral artery disease | 720 | observational longitudinal cohort study N/A |
Serum Plasma |
|
Gait Speed
SPPB |
3MSE
|
|
|
WALCS II
(McDermott, NW) |
M / F | 59 + | Peripheral artery disease | 800 | observational longitudinal cohort study N/A |
Serum Plasma |
CT
|
Gait Speed
SPPB Leg Press |
Fitbit
|
MMSE
CES-D |
| Study (PI) | Gender | Age | Disease/ Condition |
N= | Design / Intervention |
Biospecimen | Imaging | Physical | Activity Monitoring |
Cognitive / Depression |
WHO CAN REGISTER STUDY META DATA:
OAIC-Affliated Centers are eligible to submit meta data related to their studies.
HOW TO GET STARTED:
Contact the Registry Coordinator at (336) 713-7126 or PepperCenter@WakeHealth.edu with further questions.
